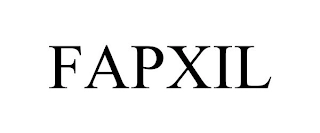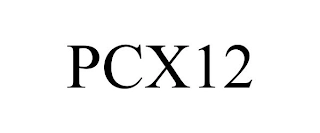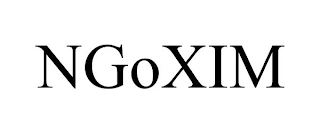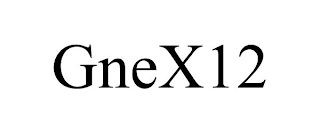Transaction Description:
OMV VACCINE AGAINST GONORRHEA - ABSTRACT GONORRHEA IS A GENITAL TRACT INFECTION CAUSED BY NEISSERIA GONORRHOEAE, WHICH CAN BE ACQUIRED REPEATEDLY AND DOES NOT NATURALLY INDUCE A STATE OF SPECIFIC PROTECTIVE IMMUNITY AGAINST REINFECTION. DESPITE PUBLIC HEALTH MEASURES, GONORRHEA PERSISTS AT AN UNACCEPTABLY HIGH FREQUENCY, AND THERE IS NO VACCINE AGAINST IT. THE CONTINUING EMERGENCE OF ANTIBIOTIC RESISTANCE THREATENS TO RENDER GONORRHEA UNTREATABLE. OUR FINDINGS HAVE REVEALED THAT N. GONORRHOEAE SUBVERTS THE IMMUNE SYSTEM FOR ITS OWN BENEFIT BY INDUCING HIGH LEVELS OF THE IMMUNO-REGULATORY CYTOKINE, INTERLEUKIN-10 (IL-10). USING THE ESTABLISHED MURINE MODEL OF VAGINAL GONOCOCCAL INFECTION, WE PROPOSE TO INVESTIGATE A NOVEL STRATEGY OF VACCINATION AGAINST N. GONORRHOEAE, EXPLOITING NEUTRALIZING ANTIBODY AGAINST IL-10 ENCAPSULATED IN BIODEGRADABLE MICRO-PARTICLES (GNEXA10®) AS ADJUVANT. AS VACCINE ANTIGEN, OUTER MEMBRANE VESICLES (OMV) DERIVED FROM A DOUBLE- MUTANT (DM) STRAIN OF N. GONORRHOEAE, DESIGNED TO REDUCE ADVERSE RESPONSES TO ADMINISTRATION IN HUMANS, WILL BE USED. WE WILL DETERMINE THE ROLE OF SERUM AND MUCOSAL ANTIBODY AND T-CELL CYTOKINE RESPONSES TO INTRA-NASAL (I.N.) IMMUNIZATION WITH DMOMV PLUS GNEXA10®, IN RESISTANCE TO CHALLENGE INFECTION WITH BOTH HOMOLOGOUS AND HETEROLOGOUS STRAINS. PERSISTENCE OF IMMUNITY AND RECALL OF MEMORY RESPONSES AT INCREASING TIME INTERVALS AFTER IMMUNIZATION WILL BE EVALUATED. FUNCTIONAL ANTIBODIES ACTIVE AGAINST N. GONORRHOEAE WILL BE ASSESSED IN VITRO. THIS WORK WILL PROVIDE PROOF-OF-PRINCIPLE FOR A VACCINE BASED ON GONOCOCCAL DMOMVS AND GNEXA10®. FUTURE SBIR PHASE II STUDIES WILL FURTHER ELUCIDATE MECHANISMS OF CROSS-PROTECTION AGAINST DIVERSE STRAINS OF N. GONORRHOEAE, AND VALIDATE THE EXPECTED APPLICABILITY OF SCALED-UP VACCINE DEVELOPMENT, EVALUATING YIELD, BIOACTIVITY, BATCH-TO-BATCH CONSISTENCY, AND SHELF STABILITY. THIS PROPOSAL IS PART OF OUR COMPANY’S RESPONSE TO THE CDC’S AND WHO’S CALLS FOR DEVELOPMENT OF NOVEL VACCINE APPROACHES IN THE FACE OF THE PUBLIC HEALTH THREAT POSED BY ANTIBIOTIC-RESISTANT N. GONORRHOEAE.